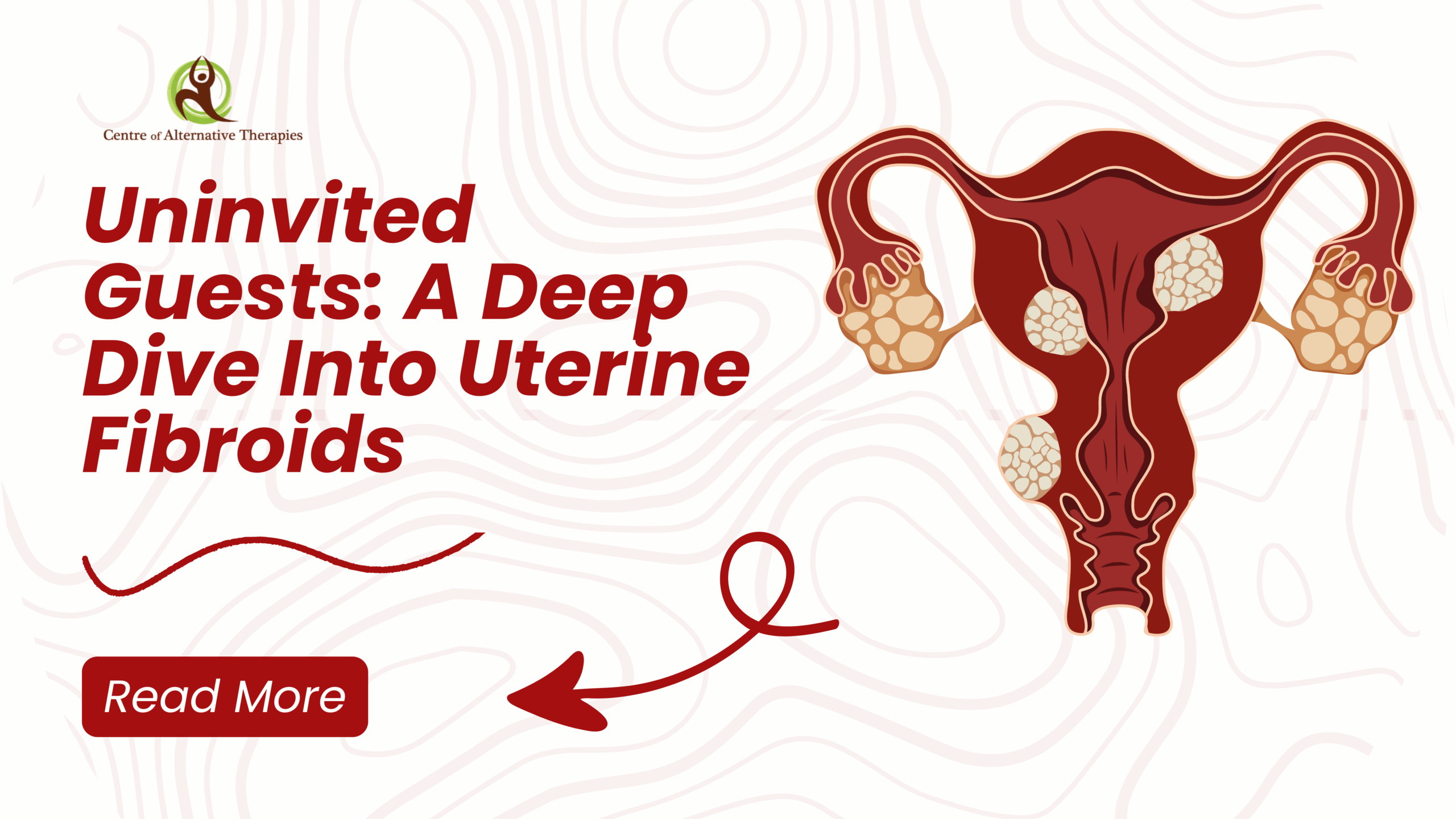
Uninvited Guest: A Deep Dive Into Uterine Fibroids
Imagine living with intense cramps, fatigue, and unpredictable bleeding and being told it’s just ‘part of being a woman.’ For many, that’s life with uterine fibroids: silent, misunderstood, and too often dismissed. Uterine leiomyomata, or fibroids, are common benign tumors that affect many women at some point in their lives.
Prevalence
Uterine fibroids are highly prevalent in Canada, with up to 70-80% of women developing them by age 50. While many women with fibroids are asymptomatic, others experience symptoms like heavy bleeding, pelvic pressure, and reproductive issues. A huge portion of fibroid-related hysterectomies in Canada are due to uterine fibroids.
Globally, prevalence of uterine fibroids has increased an estimated 78.82% in the past few decades, from 126.41 million to 226.05 million cases.
Studies have shown a notable difference in the prevalence and presentation of fibroids based on race, with Black women experiencing fibroids more often and with more severe symptoms than some other racial populations.
Uterine fibroids are one of the most common reasons for a hysterectomy, yet even when the ovaries are conserved, hysterectomies can increase risks for other health issues.
Symptoms
Women with uterine fibroids often report a decreased quality of life, as the condition can cause severe and chronic symptoms such as the following:
- Heavy or prolonged menstrual bleeding
- Abnormal uterine bleeding
- Anemia
- Pelvic pain
- Infertility
- Recurrent pregnancy loss
Causes and Risk Factors
The underlying cause of uterine fibroids and the mechanisms of their growth are not fully known, and the factors that predispose women to fibroid development continue to be studied.
Not Modifiable Risk Factors
- Age
- Race
- Genetic predisposition
- Family history
Modifiable Risk Factors
Lifestyle factors that play a role in fibroid development are:
Physical activity
- Diet
- Stress
- Smoking status
- In addition, obesity, high serum lipids, and metabolic syndrome may increase the risk of fibroids, suggesting a cardiometabolic connection.
- Low vitamin D levels as well as chronic inflammation may also play a role in fibroid formation.
- Research studies suggest that chronic exposures to environmental toxicants play a role in the development of fibroids, including exposures to cosmetic or beauty-product-related chemicals.
- Polluted drinking water
- Endocrine-disrupting chemicals (EDCs)
Diagnosis
Diagnosis typically involves:
- Pelvic Examination: A doctor may feel irregularities in the shape of the uterus.
- Imaging Tests: Ultrasound is commonly used to confirm the presence of fibroids. MRI may be employed for more detailed imaging.
- Other Tests: Hysterosonography, hysterosalpingography, or hysteroscopy may be used in certain cases to evaluate the uterus more thoroughly.
- Lab Testing
- Comprehensive Hormone Panels: Assess levels of estrogen, progesterone, and other hormones.
- Liver Function Tests: Evaluate the efficiency of detoxification pathways.
- Gut Microbiome Analysis: Identify imbalances in gut flora that may affect estrogen metabolism.
- Nutrient Deficiency Testing: Determine levels of essential vitamins and minerals.
Treatment Considerations
Healthy lifestyle factors may not only play a preventative role in fibroid risk but may also be part of an effective treatment plan. As part of a personalized therapeutic strategy to decrease uterine fibroids, the following are all components of a foundational functional medicine approach:
- Addressing hormonal imbalances that may include estrogen dominance.
- Supporting gut health and decreasing overall inflammation.
- Improving detoxification and waste elimination to help remove excess estrogen.
- Stabilizing blood sugar and insulin.
- Reducing exposure to endocrine-disrupting chemicals.
- Implementing the most effective exercise strategy for an individual patient.
- Addressing micronutrient deficiencies and prescribing a personalized nutrition intervention.
- One of the emerging non-surgical treatments for fibroids is modulating progesterone. Uterine fibroid cells themselves have been shown to alter the expression of ovarian hormone receptors. For that reason, it is important to help patients restore hormonal balance even after their fibroids have been removed and the resulting symptoms are adequately treated.
Homeopathy
Homeopathy has great scope in treating cases of heavy bleeding due to uterine fibroids. These work magnificently in overcoming a tendency of heavy bleeding by targeting to dissolve the uterine fibroids. The most suitable homeopathic medicine for treating these cases is selected after detailed analysis in every individual case. The best part of homeopathic medicines is that these work in the most natural way to treat a condition without any side-effects.
- Thlaspi Bursa Pastoris is the topmost medicine to manage cases of heavy bleeding from uterine fibroids. In cases needing it, there occurs heavy, bright red, uterine bleeding. Clots may also be present in the menstrual blood.
- Sabina this medicine is highly recommended when there is heavy bright red bleeding along with dark clots.
- Hamamelis is the most suitable medicine when there is heavy bleeding that is dark in color.
- Calcarea Carbonica: for managing early, heavy, and prolonged menses.
- Natrum Muriaticum: Considered for cases with irregular menstruation and emotional stress.
- Aurum Muriaticum Natronatum: Studied for its effectiveness in reducing fibroid size and alleviating symptoms.
- Trillium Pendulum – For Gushing Of Blood From Slight Movement
- Phosphorus – For Frequent And Heavy Periods
- Ustilago Maydis: For Dark Clotted Blood Forming Long Black Strings
- Secale Cornutum – For Heavy, Thin And Black Bleeding
- Kali Iodatum – With Heavy Painful Menses And Frequent Urging To Urinate
- Nitricum Acidum – For Heavy, Thick And Dark Coloured Bleeding
- Platina – For Dark Clotted Heavy Bleeding
- Sepia Officinalis – For Uterine Fibroids where Menses are Painful
- Fraxinus Americana – Excellent Homeopathic medicine for uterine fibroids with bearing down sensation
- Erigeron Canadensis – For Frequent Urination in case of Uterine Fibroids
Tissue Salts
Tissue salts, also known as cell salts, are mineral-based remedies that support cellular function. For uterine fibroids, the following are commonly considered:
- Calcarea Fluorica (Calc Fluor): Believed to maintain tissue elasticity and may help in managing fibroid growth.
- Calcarea Phosphorica (Calc Phos): Supports bone and tissue health, potentially beneficial in fibroid management
Ayurvedic Herbs
Ayurveda offers a holistic approach to managing uterine fibroids, emphasizing balance and detoxification. Several herbs have been traditionally used:
- Kanchanara Guggulu: A classical formulation reputed for its potential to reduce fibroid size and alleviate symptoms.
- Shigru Guggulu: Combines the benefits of Shigru (Moringa) and Guggulu, used for its anti-inflammatory properties.
- Haridra Khand: Contains turmeric, known for its anti-inflammatory and antioxidant effects, which may support fibroid management
Lifestyle Modifications
While some risk factors may not be modifiable, the development of uterine fibroids may be influenced by some modifiable lifestyle factors. Interventions that prioritize hormonal balance and reduce systemic inflammation, improve modifiable lifestyle factors, and reduce exposure to environmental toxicants are treatment approaches that should be considered for uterine fibroids. For the many women who suffer from fibroids but wish to avoid hysterectomy, these low-harm therapies may provide the relief they seek.
- Reduce Inflammation by prescribing Elimination Diet, Detox Plan, Cardiometabolic Food plan and Mito Food Plan
- Increase phytonutrient density: colored fruits, vegetables: 3 portions/d,5 portions/d,7 portions/d,9 portions/d.
- Increase EFA Omega 3
- Weight loss, decrease adiposity.
- Remove/reduce sources of endocrine disruptors: food, personal care, plasticizers.
- Reduce alcohol.
- Reduce caffeine and refined carbohydrates
- Support Gut health
- Support detox mechanisms
- Support Thyroid function
- Support Adrenal function
- Acupuncture: May help alleviate fibroid-related symptoms, though more research is needed.
- Stress Management: Techniques such as yoga and meditation can help manage stress, which may influence hormone levels.
Foods that modify Estrogen Metabolism
- Consume probiotic rich foods: yogurt/kefir, miso, natto, tempeh, Sauerkraut, Kimchi, raw pickles, fermented anything, root and ginger beers, Pulque, kombucha, fermented vegetables, buttermilk, raw whey, raw vinegars, fermented sausages, sourdough, essence bread
- Eat organic if possible
- Cruciferous vegetables broccoli, cabbage, Brussel sprouts.
- Fresh and green vegetables.
- Fruits berries, melons, citrus.
- Legumes tofu, miso, edamame, chickpeas.
- Beans
- Raw seed and nuts flax, chia
- Whole grains oats, rye berries.
- Quality protein salmon, tuna, haddock, hormone free chicken and turkey.
- Oils flax seed, olive, sesame.
- Green tea
Nutritional Supplements
Nutritional support plays a role in managing fibroids, particularly in addressing deficiencies and supporting hormonal balance:
- Curcumin: An anti-inflammatory compound that may help manage fibroid-related inflammation.
- Vitamin D 3: Deficiency has been linked to fibroid development; supplementation may help inhibit growth and can also affect hormone regulation and inflammatory processes
- Green Tea Extract (EGCG): Epigallocatechin gallate has shown potential in reducing fibroid size and improving symptoms.
- Iron: Essential for individuals experiencing heavy menstrual bleeding to prevent anemia.
- Vitamins B6, meB12, 5MTHFolic Acid and choline to promote bile synthesis and modify estrogen metabolism. May aid in hormone regulation and alleviate menstrual discomfort associated with fibroids
- Phytoestrogens and Lignans (Flax) to increase Sexual Hormone Binding Globulin (SHBG) to bind circulating estrogens 10-30 g/d
- Probiotics: Lactobacillus acidophilus and Bifidobacteria lactis for Beta glucuronidase deactivation.
- Insoluble fiber (lignans) to improve intestinal excretion transit time.
- Glucosinolates: Indole 3Carbinol(I3C). Indole-3-carbinol (I3C), a naturally occurring compound found in cruciferous vegetables, has shown potential anti-fibrotic and anti-migratory effects on uterine fibroids in vitro, suggesting it may play a role in reducing their growth and spread and modifies the 2-hydroxyestrone (2-OHE1) to 16α-hydroxyestrone (16α-OHE1) E1 Ratio.
- Diindolylmethane (DIM): support healthy estrogen metabolism and modifies the 2-hydroxyestrone (2-OHE1) to 16α-hydroxyestrone (16α-OHE1) E1 Ratio.
- Magnesium modifies estrogen metabolism and can also affect hormone regulation and inflammatory processes
- Vitamin C modify estrogen metabolism.
- Fish Oils modify estrogen metabolism and can also affect hormone regulation and inflammatory processes
- Chromium modifies estrogen metabolism.
- Calcium d-glucarate modify estrogen metabolism.
- Inositol, particularly D-chiro-inositol and myo-inositol, has shown potential in managing uterine fibroids, particularly when combined with other supplements like vitamin D and green tea extract.
- N-acetyl cysteine Preliminary clinical research shows that taking N-acetyl cysteine 600 mg daily for 12 weeks reduces the volume of uterine fibroids by about 25%, compared with a reduction of about 1% with placebo. N-acetyl cysteine also reduces painful and heavy menstrual bleeding when compared with placebo.
Modification of 2-hydroxyestrone (2-OHE1) to 16α-hydroxyestrone (16α-OHE1) E1 Ratio
The 2-hydroxyestrone (2-OHE1) to 16α-hydroxyestrone (16α-OHE1) ratio, often referred to as the 2/16 ratio, is a marker of estrogen metabolism. While the 2/16 ratio has been studied in relation to breast cancer risk, there is less research specifically focusing on its connection to uterine fibroids. Some studies suggest that a lower 2/16 ratio may be associated with a higher risk of certain hormone-related conditions, including uterine fibroids. A higher 2/16 ratio may indicate a preference for the “safer” 2-hydroxylation pathway, while a lower ratio may suggest more of the potentially more harmful 16α-hydroxylation pathway.
There are several foods, herbs and supplements that can increase this ratio:
- Flax Seed the recommended quantity is 10-30 g/d
- Omega 3
- Soy isoflavones
- Indole 3Carbinol(I3C)
- Diindolylmethane (DIM)
- Rosemary
- Turmeric
- Kudzu
- Strenuous Exercise
- Weight loss
Aromatase Inhibitors
Aromatase is a key enzyme in the body which regulates the conversion of androgens to estrogens. When testosterone levels are low, or estrogen levels are high, lowering or inhibiting aromatase activity may help re-establish healthy hormone levels. There are several foods, herbs and supplements that may inhibit or lower aromatase activity.
- Sources of Lignans
- Cashew
- Flax seed
- Chickpeas
- Cranberry
- Sunflower seeds
- Oats
- Pineapple
- Rye
- Buckwheat
- Eggplant
- Sesame seeds
- Asparagus
- Millet
- Oranges
- Coffee
Selective Estrogen Enzyme Modulators (SEEMS)
There are several foods, herbs and supplement that interact with estrogen receptors in the body, but unlike estrogen, they can either mimic or block its effects in different tissues. They can act as agonists (mimicking estrogen) in some tissues and antagonists (blocking estrogen) in others
- Resveratrol
- Catechins
- Curcumin
- Gogi berries
- Omega 3
- Isoflavones
- Hesperidin
- I3C
- DIM
- Quercetin
- Progestogens
- Sulfamates
Modifiers that increase Sexual Hormone Binding Globulin (decrease Estrogen effect)
- Vitamin D
- Low fat diet
- Low protein vegetarian diet
- Treat Hypothyroidism and Obesity
Modifiers that decrease Sexual Hormone Binding Globulin (increase Estrogen effect)
Sex Hormone-Binding Globulin, a protein produced by the liver that binds to and transports sex hormones like testosterone and estradiol in the blood. It plays a crucial role in regulating the availability of these hormones to target tissues, influencing their activity in the body.
- Stinging Nettle
- EPA/DHA
- Whey protein
- Testosterone therapy
- Exercise-GH
Conclusion
It’s crucial to approach the management of uterine fibroids with a comprehensive plan that includes medical consultation, especially when considering alternative therapies. Regular monitoring and individualized treatment strategies are key to effective management.
The Centre of Alternative Therapies approach to uterine fibroids focuses on identifying and addressing the root causes of fibroid development, emphasizing personalized care through hormonal balance, detoxification, nutrition, and lifestyle modifications. This integrative strategy aims to reduce fibroid size, alleviate symptoms, and improve overall reproductive health.
References
Aghaamoo, S., Zandbina, A., Saffarieh, E., & Nassiri, S. (2021). The effect of N-acetyl cysteine on the volume of uterine leiomyoma: A randomized clinical trial. International Journal of Gynaecology and Obstetrics: The Official Organ of the International Federation of Gynaecology and Obstetrics, 154(3), 521–525. https://doi.org/10.1002/ijgo.13611
AlAshqar, A., Reschke, L., Kirschen, G. W., & Borahay, M. A. (2021). Role of inflammation in benign gynecologic disorders: From pathogenesis to novel therapies†. Biology of Reproduction, 105(1), 7–31. https://doi.org/10.1093/biolre/ioab054
Bariani, M. V., Rangaswamy, R., Siblini, H., Yang, Q., Al-Hendy, A., & Zota, A. R. (2020). The role of endocrine-disrupting chemicals in uterine fibroid pathogenesis. Current Opinion in Endocrinology, Diabetes, and Obesity, 27(6), 380–387. https://doi.org/10.1097/MED.0000000000000578
Bougie, O., Bedaiwy, M. A., Laberge, P., Lebovic, G., Leyland, N., Atri, M., & Murji, A. (2020). Quality of ultrasonography reporting and factors associated with selection of imaging modality for uterine fibroids in Canada: Results from a prospective cohort registry. Canadian Medical Association Open Access Journal, 8(3), E506–E513. https://doi.org/10.9778/cmajo.20200004
Brewster, L. M., Haan, Y., & van Montfrans, G. A. (2022). Cardiometabolic Risk and Cardiovascular Disease in Young Women With Uterine Fibroids. Cureus, 14(10), e30740. https://doi.org/10.7759/cureus.30740
Gaston, S. A., James-Todd, T., Harmon, Q., Taylor, K. W., Baird, D., & Jackson, C. L. (2020). Chemical/straightening and other hair product usage during childhood, adolescence, and adulthood among African-American women: Potential implications for health. Journal of Exposure Science & Environmental Epidemiology, 30(1), 86–96. https://doi.org/10.1038/s41370-019-0186-6
Green, T., See, J., Schauch, M., Reil, J., Glover, M., Brix, J., Gerry, A., Li, K., Newman, M., Gahler, R. J., & Wood, S. (2023). A randomized, double-blind, placebo-controlled, cross-over trial to evaluate the effect of EstroSense® on 2-hydroxyestrone:16α-hydroxyestrone ratio in premenopausal women. Journal of Complementary & Integrative Medicine, 20(1), 199–206. https://doi.org/10.1515/jcim-2022-0301
Hammarstrand, S., Jakobsson, K., Andersson, E., Xu, Y., Li, Y., Olovsson, M., & Andersson, E. M. (2021). Perfluoroalkyl substances (PFAS) in drinking water and risk for polycystic ovarian syndrome, uterine leiomyoma, and endometriosis: A Swedish cohort study. Environment International, 157, 106819. https://doi.org/10.1016/j.envint.2021.106819
Katon, J. G., Plowden, T. C., & Marsh, E. E. (2023). Racial disparities in uterine fibroids and endometriosis: A systematic review and application of social, structural, and political context. Fertility and Sterility, 119(3), 355–363. https://doi.org/10.1016/j.fertnstert.2023.01.022
Lou, Z., Huang, Y., Li, S., Luo, Z., Li, C., Chu, K., Zhang, T., Song, P., & Zhou, J. (2022). Global, regional, and national time trends in incidence, prevalence, years lived with disability for uterine fibroids, 1990-2019: An age- period-cohort analysis for the Global Burden of Disease 2019 study. https://doi.org/10.21203/rs.3.rs-2354839/v1
Luthra, J., Chattopadhyay, A., Nayak, C., Bhar, K., Singh, N., Koley, M., & Saha, S. (2023). Aurum Muriaticum Natronatum in Treatment of Uterine Fibroids: A Randomized Trial. Journal of Applied Sciences and Clinical Practice, 4, 31. https://doi.org/10.4103/jascp.jascp_38_22
MacLean, J. A., & Hayashi, K. (2022). Progesterone Actions and Resistance in Gynecological Disorders. Cells, 11(4), 647. https://doi.org/10.3390/cells11040647
Marsh, E. E., Al-Hendy, A., Kappus, D., Galitsky, A., Stewart, E. A., & Kerolous, M. (2018). Burden, Prevalence, and Treatment of Uterine Fibroids: A Survey of U.S. Women. Journal of Women’s Health (2002), 27(11), 1359–1367. https://doi.org/10.1089/jwh.2018.7076
Miriello, D., Galanti, F., Cignini, P., Antonaci, D., Schiavi, M. C., & Rago, R. (2021). Uterine fibroids treatment: Do we have new valid alternative? Experiencing the combination of vitamin D plus epigallocatechin gallate in childbearing age affected women. European Review for Medical and Pharmacological Sciences, 25(7), 2843–2851. https://doi.org/10.26355/eurrev_202104_25537
Mohammadi, R., Tabrizi, R., Hessami, K., Ashari, H., Nowrouzi-Sohrabi, P., Hosseini-Bensenjan, M., & Asadi, N. (2020). Correlation of low serum vitamin-D with uterine leiomyoma: A systematic review and meta-analysis. Reproductive Biology and Endocrinology: RB&E, 18(1), 85. https://doi.org/10.1186/s12958-020-00644-6
Pavone, D., Clemenza, S., Sorbi, F., Fambrini, M., & Petraglia, F. (2018). Epidemiology and Risk Factors of Uterine Fibroids. Best Practice & Research. Clinical Obstetrics & Gynaecology, 46, 3–11. https://doi.org/10.1016/j.bpobgyn.2017.09.004
Qin, H., Lin, Z., Vásquez, E., Luan, X., Guo, F., & Xu, L. (2021). Association between obesity and the risk of uterine fibroids: A systematic review and meta-analysis. Journal of Epidemiology and Community Health, 75(2), 197–204. https://doi.org/10.1136/jech-2019-213364
Sohn, G. S., Cho, S., Kim, Y. M., Cho, C.-H., Kim, M.-R., Lee, S. R., & Working Group of Society of Uterine Leiomyoma. (2018). Current medical treatment of uterine fibroids. Obstetrics & Gynecology Science, 61(2), 192–201. https://doi.org/10.5468/ogs.2018.61.2.192
Tinelli, A., Panese, G., Licchelli, M., Morciano, A., Pecorella, G., & Gambioli, R. (2024). The impact of epigallocatechin gallate, vitamin D, and D-chiro-inositol on early surgical outcomes of laparoscopic myomectomy: A pilot study. Archives of Gynecology and Obstetrics, 309(3), 1021–1026. https://doi.org/10.1007/s00404-023-07324-x
Vahdat, M., Allahqoli, L., Mirzaei, H., Giovannucci, E., Salehiniya, H., Mansouri, G., & Alkatout, I. (2022). The effect of vitamin D on recurrence of uterine fibroids: A randomized, double-blind, placebo-controlled pilot study. Complementary Therapies in Clinical Practice, 46, 101536. https://doi.org/10.1016/j.ctcp.2022.101536
Vilos, G. A., Allaire, C., Laberge, P.-Y., Leyland, N., & SPECIAL CONTRIBUTORS. (2015). The management of uterine leiomyomas. Journal of Obstetrics and Gynaecology Canada: JOGC = Journal d’obstetrique et Gynecologie Du Canada: JOGC, 37(2), 157–178. https://doi.org/10.1016/S1701-2163(15)30338-8
Wadhwa, B., & Kureel, S. S. (2024). Homeopathic Management of Bulky Uterus with Uterine Fibroid in a 40-year-old Patient: An Evidence-Based Case Report. Homeopathy: The Journal of the Faculty of Homeopathy. https://doi.org/10.1055/s-0044-1790513
Yerushalmi, R., Bargil, S., Ber, Y., Ozlavo, R., Sivan, T., Rapson, Y., Pomerantz, A., Tsoref, D., Sharon, E., Caspi, O., Grubsrein, A., & Margel, D. (2020). 3,3-Diindolylmethane (DIM): A nutritional intervention and its impact on breast density in healthy BRCA carriers. A prospective clinical trial. Carcinogenesis, 41(10), 1395–1401. https://doi.org/10.1093/carcin/bgaa050